An Austrian biotech company is developing cell therapies for incontinence cure that could help millions of sufferers live an active life again
Imagine you rush to the bathroom but you don’t make it in time. A scary idea, but unfortunately a reality for many people. In Europe alone, about 20 million adults suffer from faecal incontinence. Urinary incontinence is even more common.
Innovacell, an Austrian biotech company, is developing cell therapies that have the potential to solve this problem. The therapy injects a patient´s own muscle precursor cells into the person´s damaged and/or weakened sphincter muscle, helping it regenerate and regain its proper function.
To speed up the final phase of clinical trials, Innovacell received a €15 million venture loan from the European Investment Bank in December 2021. The money will help the company stay ahead of the competition after development slowed during the COVID-19 pandemic.
“There is one US company that works on this kind of therapy. They are at a minimum five years behind us in development,” says Ekkehart Steinhuber, the company’s chief executive. “The loan was material in that it helped assure investors that we had sufficient funds for the foreseeable future.”
Incontinence cure improves quality of life
Incontinence is more prevalent among the elderly. It is the second most common reason why people are placed in nursing homes. However, younger people suffer as well. Women, for example, can suffer trauma to the sphincter during childbirth.
“The field is underestimated. Patients often don’t go to the doctor because of the stigma attached to the illness,” says Valeria Iansante, a life science specialist at the European Investment Bank. Those affected feel ashamed and end up placing limits on their lives so they can maintain easy access to a bathroom. They avoid sports and any other activities. Quite often, they fall into depression.
So far, there is “not really much people can do to contain the leak,” Iansante says. Existing treatments range from dietary change and physiotherapy to medication, electrical stimulation and various forms of surgery. Some of them are massively invasive and often don’t have a lasting effect.
One-time injection for a long-term cure
Innovacell has chosen a different approach. The company takes muscle tissue the size of a cherry pit from the patient’s chest muscle. This is done under the arm to leave no visible scarring. The process isolates stem cells from the tissue and develops them into muscle precursor cells, which are then cultivated to multiply. These cells are re-injected in the patient’s sphincter, where they help the muscle regenerate and regain its function.
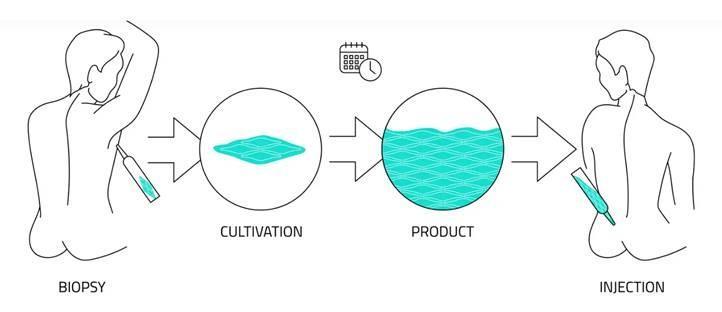
The company uses patented technology to implant the cells with a very small needle. The procedure, which is guided by ultrasound, is minimally invasive and very precise. And it potentially provides a permanent cure: “We have data from patients treated eight years ago,” says Chief Executive Steinhuber, who has been with the company since 2009. “The effect lasts.”
EU help on the run to approval
Innovacell has three product candidates:
- ICEF15 treats “urge faecal incontinence,” where patients feel a sudden need to go to the bathroom but are unable to reach it in time;
- ICEF16 addresses “passive faecal incontinence,” where patients feel no sensation before soiling themselves;
- ICES13 is a therapy for “stress urinary incontinence,” where coughing, sneezing or physical exertion can lead to unwanted loss of urine.
ICEF15, the company’s lead candidate, has entered phase III, the last phase of clinical trials before regulatory approval. At this stage, the company needs to invest huge funds. “Such a study costs tens of millions of euros,” Steinhuber says.
Raising money at this stage and in this sector is not easy in Europe. That leads many companies to seek financing in the United States or Asia, often resulting in an exodus of science from Europe.
“There is a lack of interest by European investors for cell therapies,” says Cyril Teixeira Da Silva, an investment officer at the European Investment Bank who helped to structure the loan to Innovacell. “But we would like to keep this innovation in Europe.”
As the EU bank, the European Investment Bank was able to offer Innovacell venture funding backed by the European Guarantee Fund. The fund was set up to help companies hit by the pandemic. “It is still a risky investment,” Teixeira Da Silva says. “Without the guarantee, it would not have been financeable yet. We would at least have had to wait for positive results in phase III.”
Japan holds promise for regenerative medicine
Innovacell sees a big opportunity in Japan, whose older population makes it a dynamic market for incontinence treatments. The company is doing phase-III trials there as well to get its product approved. Japan is attractive because the potential of regenerative therapies is recognized. That makes it easier for companies developing these research-intensive treatments to charge higher prices.
At the same time, doctors are well trained in incontinence, which is less of a taboo than in Europe. Japan also has national ambitions to lead the world in regenerative therapies, spurred by Shinya Yamanaka’s winning the Nobel Prize for stem cell research in 2012.
By financing Innovacell, which started in 2000 as a spin-off of the Medical University of Innsbruck, the Bank is enabling the company to export its science globally, benefitting people in the European Union and beyond.
“They can be considered the future of regenerative medicine,” says the EIB’s Iansante. “There is a high medical need and the benefits for patients could be so large. This is why we are bringing EU funds to this company.”
